
BCP stands for “bacterioferritin comigratory protein,” and members of the BCP-PrxQ subfamily include the bacterial BCP proteins (Jeong et al. 2000) and the plant PrxQ (Kong et al. 2000) proteins. These proteins are predominantly bacterial, although they are present in some eukaryotes including yeast and plants but excluding mammals (Nelson et al. 2011). To date, the BCP-PrxQ subfamily contains the only Prx proteins shown to function as monomers. Although most are reportedly monomeric, there are two examples of dimeric subfamily members containing an A-type dimer (PDB identifiers 2cx4, 2ywn). Current information suggests that BCP-PrxQ subfamily members can function as either atypical 2-Cys (α-group) or 1-Cys (β-group) (Wakita et al. 2007). While the prototypical location for the CR is found five residues after the CP in helix α2, the CR in Xanthomonas campestris BCP is found in helix α3 (the prototypical location for Tpx subfamily members)(Liao et al. 2009).
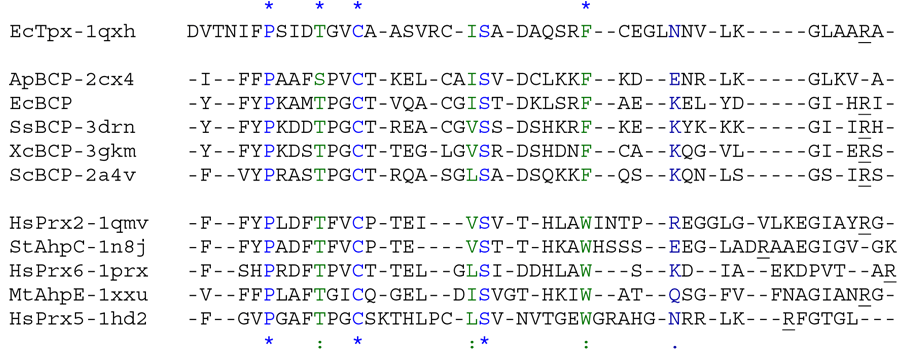
Aligned active site signatures (from DASP; Nelson et al. 2011) from the BCP-PrxQ subfamily (center group) and one representative each of the other five subfamilies (upper and lower group). Key residues of the active site signatures are noted by asterisks along the top. Shown below is the unrooted tree generated by Phylip’s Drawtree using the SDSC Biology Workbench tools.
8 structures, 5 proteins
| PDB identifiers | Name | Species |
|---|---|---|
| 2a4v | BCP | Saccharomyces cerevisiae |
| 2cx4, 2cx3 | BCP | Aeropyrum pernix |
| 2ywn | Prx-like | Sulfolobus tokodaii |
| 3drn | BCP-1 | Sulfolobus solfataricus |
| 3gkk, 3gkn, 3gkm | BCP | Xanthomonas campestris |