
Peroxiredoxins (Prxs) (EC 1.11.1.15) are ubiquitous and abundant proteins that are important for antioxidant defense and regulate cell signaling pathways. Prxs are cysteine-based peroxidases found in all organisms with the single exception, to our knowledge, of Borrelia burgdorferi and other Borrelia species. Mammals express six different Prxs from distinct genes (Prx I-Prx VI), and E. coli expresses three (AhpC, Tpx and BCP). The broad distribution of Prxs and the high levels of expression suggest they are both an ancient and important enzyme family; they are among the top ten most abundant E. coli proteins (Link et al. 1997), and typically comprise ~0.1 to 1% of the total cellular proteins in mammalian cells (Wood et al. 2003; Veal et al. 2007). In bacteria, the main Prx of E. coli, AhpC, was found to be the primary enzyme responsible for the reduction of endogenously-generated H2O2, with catalase becoming important for protection against intracellular H2O2 levels around 5 μM or higher (Seaver and Imlay 2001). In plants, Prxs protect the photosynthetic apparatus from oxidative damage (Rouhier and Jacquot 2005). In mammals, Prxs have been implicated in many studies in regulating such processes as cell proliferation, differentiation, cell death, and intracellular signaling (Kang et al. 2005; Veal et al. 2007; Poole and Nelson 2008). Prxs have also been shown to be aberrantly expressed in cancer and to impact the radiation sensitivity of cells (Zhang et al. 2009).
Subfamilies of Prxs have been defined based on previously published structural characterizations and our own hierarchical clustering of the aligned functional site signatures (Hall et al. 2010).
For simplicity and clarity, we have used the following names for subfamily classes:
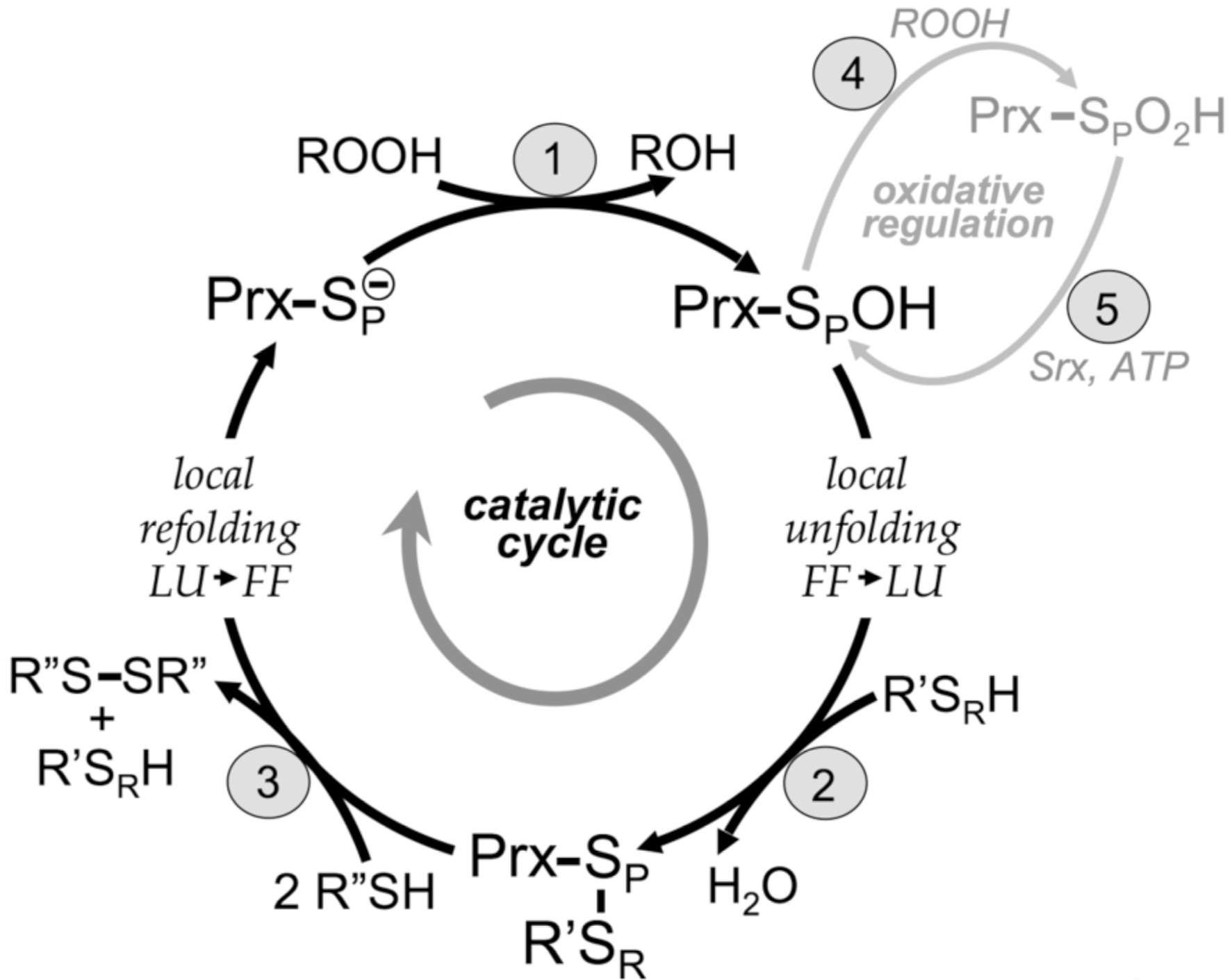
Peroxide reduction by Prxs involves three main chemical steps of (1) peroxidation, (2) resolution, and (3) recycling, as described in Hall et al. (2010). Two distinct protein conformations are involved in the cycle: FF (fully folded, active site intact) and LU (locally unfolded, disulfide between the CP and the CR). The local unfolding event is required for disulfide bond formation in step 2 as is the local refolding event to reform the peroxide-binding active site after the disulfide is reduced in step 3. Oxidative regulation (gray, steps 4 and 5) is seen in sensitive, eukaryotic floodgate-type 2-Cys Prxs. Inactivation of the Prx by overoxidation of the CP (step 4) is peroxide dependent. The inactivated form can be rescued through an ATP-dependent reaction catalyzed by sulfiredoxin (Srx) (step 5). The generic Prx is represented as a monomer with SP designating the sulfur atom of the CP. The CR (from R' with SR designating the sulfur atom) can be supplied by a different protein or small molecule (1-Cys mechanism) or by a second Cys within the same Prx either on the same chain or on the other subunit of a B-type dimer (2-Cys mechanism). Different proteins, including Trx and AhpF, have been identified as R" in step 3.